UChicago Pritzker Molecular Engineering Prof. Y. Shirley Meng’s Laboratory for Energy Storage and Conversion has created the world’s first anode-free sodium solid-state battery.
With this research, the LESC – a collaboration between the UChicago Pritzker School of Molecular Engineering and the University of California San Diego’s Aiiso Yufeng Li Family Department of Chemical and Nano Engineering – has brought the reality of inexpensive, fast-charging, high-capacity batteries for electric vehicles and grid storage closer than ever.
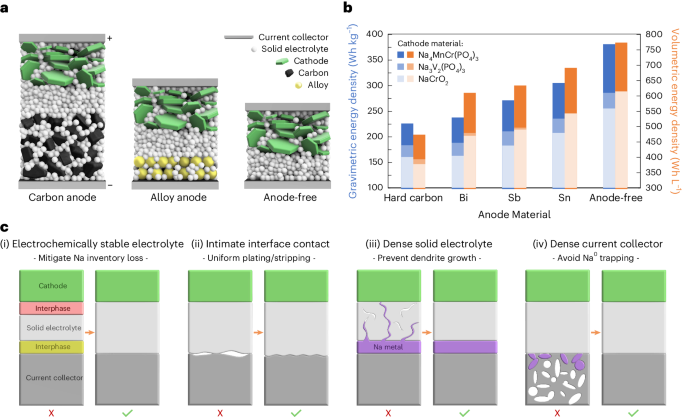
“Although there have been previous sodium, solid-state, and anode-free batteries, no one has been able to successfully combine these three ideas until now,” said UC San Diego PhD candidate Grayson Deysher, first author of a new paper outlining the team’s work.
The paper, published today in Nature Energy, demonstrates a new sodium battery architecture with stable cycling for several hundred cycles. By removing the anode and using inexpensive, abundant sodium instead of lithium, this new form of battery will be more affordable and environmentally friendly to produce. Through its innovative solid-state design, the battery also will be safe and powerful.
This work is both an advance in the science and a necessary step to fill the battery scaling gap needed to transition the world economy off of fossil fuels.


How do you connect it to a circuit without an anode?
I am wondering the same thing
It’s a misleading name.
Normally the current collector is always coated in something such as lithium, carbon or something else. In this case, the anode has just the current collector, and nothing else. That’s why it’s not actually called an anode, even though you can connect it to an external circuit. When you charge the battery, you will get the familiar anode anyway.